Identification of Cryovessels
Monitoring cooling tanks safeguards products and intermediates, even without a permanent database connection
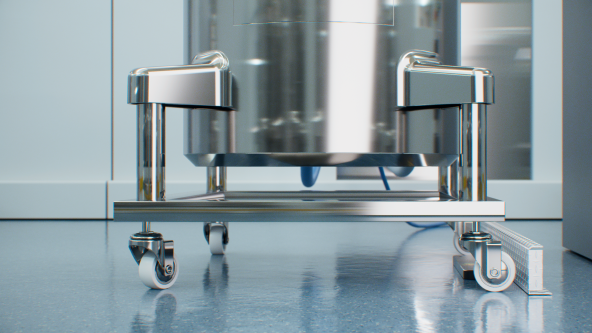
In the pharmaceutical industry, cryovessels or other active cooling containers that are transported from one facility to another are frequently used.DIN A4 files, ID sheets and data matrix codes are normally used to identify these containers so that the monitoring of these containers during transportation is not possible. In the event of an accident, a clear allocation of the cryovessel and its content is not possible. Turck's BL ident RFID system enables clear and seamless monitoring during production, transportation and storage.
YOUR BENEFITS
- Fail-safe identification with the BL ident RFID system prevents allocation errors
- Reliable identification even with inaccurate vessel positioning thanks to particularly wide HF read/write heads with a large range
Simple positioning of the vessel saves time
Automated identification solutions must be easy to use. The TNLR-Q80L400-H1147 read/write head is particularly wide and thus makes it possible to read the tag reliably without any lengthy position adjustment necessary. Large read/write ranges of up to 30 centimeters and more are possible when used in conjunction with the right tag.
Improved process reliability
The value of the cultures in cryovessels and cooling containers can quickly run into the hundreds of thousands. RFID-based identification solutions ensure that processes are only started when it is certain that the right container is in the required position. It is also possible to verify that containers have been sterilized in time.
Quality reports at the touch of a button
Complete process documentation is much easier to provide as all the necessary data is available in the system. Reliance on the diligence of responsible colleagues is no longer necessary and full documentation can be provided without the need for personnel to be present. Quality reports for FDA and other test authorities can thus be created without any major manual effort.
- Automotive
- Modular Flexibility and Safety in Filter Production
- Smart Cable Prevents Machine Downtimes
- Inductive Couplers Ensure Precise Material Feed
- Assembly Management with a Direct MES Connection
- Car Body Identification in Automobile Production
- Reliable Skid Detection in Automotive Production
- Error-free JIS Order Picking for Bumpers
- Laser Sensor Detects Shiny Cylinders in Battery Production
- RFID Bus Mode Ensures Quality of Lithium-Ion Batteries
- Level Measurement in Dip Coating Line
- Level Control in Central Lubrication System
- Quality Assurance on the Gluing Robot
- Tilt angle sensor accelerates platform alignment
- Motor Control with Condition Monitoring
- IP67 Hybrid Module Processes Safety Signals
- Decentralized monitoring of cooling stations
- Robot welding cells networked with Ethernet
- Monitoring the cooling circuit on welding clamps
- Flow monitoring in drum washers
- Press Shop – Sheet Metal Thickness Measurement
- Condition Monitoring of Motors as a Retrofit
- Cloud-based Level Monitoring
- Press Shop – Tool Identification
- Body Shop – Welding Nut Detection
- Paint Shop – Skid Identification
- Final Assembly – Robot Assembly
- Powertrain – Contactless Verification
- Body Shop – Area Guarding
- Item-level Detection with UHF RFID
- Supply of cooling lubricant in machine tools
- Press Shop: Controlling hydraulic pressure
- Measuring process pressure on scissor lifts
- Body Shop – Automotive Welding Tip Inspection
- Body Shop – Manual Load Weld Cell
- Final Assembly – Black Parts Detection
- Final Assembly – Moonroof Detection
- Final Assembly – Long-Range Inspection
- Powertrain – Rubber Washer Detection
- Powertrain – Part-In-Place Detection
- Electric Vehicle Detection
- Vehicle Detection in a Self-Serve Car Wash
- Success Stories
- RFID Solution for Data Acquisition in Stator Production
- Automation and Control of Hydrogen Filling Stations
- Modular Plant for Flexible and Efficient Production
- Laser Sensor Detects Black Bumpers in Assembly Cell
- Decentralized Safety Technology for Modular Production
- Modular Conveyor System
- RFID and I/O modules for Safe Tool Changes
- RFID Guides AGV in Suspension Production
- IO-Link Wired Silencer Production
- Workpiece Carrier Identification in Rear Vent Production
- Weld Nut Sensing
- RFID Traceability
- RFID in Engine Production
- Bumper Production with Identification
- Solutions for Paint Shops
- Welding and Assembly Sensors
- Angle Sensors for Assembly Systems
- Tool Identification
- Pick-to-Light Improves Cockpit Production
- RFID Identification of Injector Nozzles
- RFID in the Body Shop
- IO-Link Eases Differential Gear Production
- Chemical
- excom I/O System Enables Safe Hydrogen Liquefaction
- Decentralized Automation in Ex Areas
- RFID Control of Tube Connections in the Ex Area
- Ethernet Signal Connection in the I&C Room
- Ethernet-based Automation of Modular Skids
- Ex Isolation in Modular Process Plants
- Detection of Pigs
- Remote Signal I/O
- Easy Connection of Field Devices
- Signal Processing with System I/O in the Control Cabinet
- Signal Separation with Interface Technology in the Control Cabinet
- Identification of Hose Connections
- Efficient Monitoring of Cabinets in the Field
- Monitoring of Quarter Turn Actuators
- Planning and Assembly of System Solutions
- Success Stories
- Control Cabinet Monitor for Transmission of Condition Data
- Record Silo Fill Levels in Real Time Thanks to Sensor-to-Cloud
- Efficient Cooling of Industrial Furnaces
- I/O System Excom Creates Space in the I&C Rooms
- Zone 2 and 22 RFID
- Efficient Testing Control
- Intrinsically Safe Field Communication
- Process Control System Partnership
- Hazardous Area Remote I/O
- Dual Valve Position Feedback
- Flexibility with Fieldbus
- Asset Management with Remote I/O
- Correct Positioning with RFID in Carbide Production
- Compact Ex Protection
- Energy
- Capacitive Sensor Detects Point Level in Pellet Heating System
- RFID System Identifies Solar Cell Carriers
- Decentralized I/O System for Hazardous Areas at H2 Refueling Stations
- Decentralized I/O Solution in Ex Zone for H2-Fueling Station
- I/O Module Facilitates Setup and Mobile Use of Fuel Cell Test Stand
- Wind Turbine Rotor Positioning
- IP67 I/O in Coal Production
- UHF RFID Identifies Switch Gear
- Remote I/O in Biogas Plant
- Food and Beverage
- RFID Tracking Reduces Food Waste in Ice Cream Production
- Condition Monitoring Sensor Automates Climate Control
- Condition Monitoring of Control Cabinets
- Condition Monitoring in Storage Rooms
- Dough Thickness Control in Rolling Machines
- Identification of Food Containers
- Container Check
- Quick Sensor Replacement in Beverage Plants
- Detection of Pipe Elbows
- Identification of Chocolate Moulds
- Success Stories
- Cloud-based Maintenance for Steam Generators
- RFID Support Enables Track and Trace in Food Production
- Reliable Linear Position Detection in Ex Zone 22
- Decentralized Control Modules in Coldstore
- Track and Trace in Meat Production with RFID
- Contact-free Encoder in Potato Production
- UHF RFID in Food Distribution Center
- RFID for Chocolate Production
- Distributed I/O for Food Equipment
- Remote I/O for Distilleries
- RFID and Autoclaves
- IP67 Power Supplies for Conveyors
- Bottle Detection with Camera
- Logistics
- UHF RFID Tunnel for Goods Identification on Conveyor Belts
- Preventing Package Jams in the Logistics Center
- Height Control and 3D Spatial Monitoring on Autonomous Forklift Trucks
- Decentralized Control of Conveyor Modules
- Tracking Big Bags with RFID
- Distance Detection in Container Cranes
- Access Control for Protected Areas
- Decentralized Muting of Electro-sensitive Protective Equipment
- I/O Blocks Control Roller Conveyor Modules
- Container Check
- Fast Tag Detection at Warehouse Gates
- Item-level Detection with UHF RFID
- Preventive Maintenance on Conveyor Belts
- Detection of Transport Containers
- Level Detection in Vessels
- Identification of Cryovessels
- Identification of Mobile Containers with Handheld Devices
- Identification of Food Containers
- Tier 1 – Bumper Identification
- Condition Monitoring in Storage Rooms
- Collision Protection on Reach Stackers
- Success Stories
- Efficient Order Picking with Pick-to-Light System
- Efficient Solution for the Digitalization of Conveyor Technology
- Logistics: RFID Reduces Error Quota by 99 Percent
- RFID: ROI Achieved After Three Avoided Delivery Errors
- Reliable AGV Control through Sensor-based Complete Solution
- Efficient Truck Navigation in the Limited Maneuvering Area
- Sustainable Tracking of RTIs thanks to RFID
- RFID Solution for Error-Proof Material Logistics
- RFID with HF Bus Mode Eases Seed Storage
- Pick-by-Light accelerates manual logistics by over 60 percent
- Pick-by-light Solution Facilitates Assembly Processes
- RFID Enables Unmanned Store at Major Building Site
- I/O and Safety Modules Increase Throughput in Intralogistics
- Shipment Tracking for Raw Materials
- RFID-based Shipment Control Minimizes Errors
- RFID-based Tracking of Inbound and Outbound Materials
- Decentralized UHF RFID Solution
- Contact-free Encoder in Potato Production
- Decentralized Control Modules in Coldstore
- Speed Control via Radar Sensor QT50
- IP67 Power Supplies for Conveyors
- Modular Conveyor System
- RFID Solution for Warehouse
- RFID Guides AGV in Suspension Production
- RFID Identifies Pharmaceuticals
- UHF RFID in Food Distribution Center
- Autonomous Parking Assistance for Trucks
- Mobile Equipment
- Animal and Object Detection on the Combine Harvester
- Condition Monitoring Sensor Automates Climate Control
- Automatic Slope Compensation
- Distribution Lines for Field Sprayers
- Angle Measurement on a Field Sprayer
- Material Flow Monitoring on a Combine Harvester
- Determining the Boom Angle Position
- Equipment Compartment Illumination on Fire Engines
- Two-Axis Tilt Measurement on a Combine Harvester
- Collision Protection on Reach Stackers
- Success Stories
- RFID Solution with Smart Forklifts in Autombile Production
- Safe Remote Maintenance of Irrigation and Drainage Pumps
- Access Control with RFID System
- Selective Asparagus Harvester
- Position Measurement with RFID and Encoder
- Speed Control via Radar Sensor QT50
- RFID Guides AGV in Suspension Production
- Block I/O Modules on Super Yacht
- Wear-free encoder on hopper dredger
- I/O for Dust Suppression
- Cabinet Cooling
- Quick Disconnect Connectivity
- Automation Solutions for Extreme Cold
- Remote I/O for Cranes
- Rugged Heavy Metal Lifting
- Rollercoaster Positioning
- Mobile Machinery Solutions
- Exact Height Positioning
- Critical Angle Sensing
- Angle Sensor Detects Platform Lift
- Oil and Gas
- Packaging
- Decentralized RFID Package Verification
- Identification of Printing Color Cartridges
- Reliable Operation of Machines
- Container Check
- Monitoring of Caps in Filling Lines
- Monitoring Changeover Processes
- Identification of Test Bottles
- Level Monitoring of Ground Coffee
- Level Detection in Vessels
- Detection of Transport Containers
- Success Stories
- Pharma
- End-to-End Sample Tracking with RFID
- RFID Control of Tube Connections in the Ex Area
- Decentralized Package Verification
- Automate Modular Skids
- Pharmaceutical Skids with Decentralized I/O Technology
- Ex Isolation in Modular Process Plants
- Control of Valve Interfaces
- Monitoring of Quarter Turn Actuators
- Detection of Pipe Elbows
- Remote Signal I/O
- Planning and Construction of Super Skids
- Easy Connection of Field Devices
- Identification of Cryovessels
- Identification of Mobile Containers
- Identification of Mobile Containers with Handheld Devices
- Securing Hose Connections for Preliminary Products
- Identification of Hose Connections in Sterile Areas
- Identification of Big Bags and Bioreactors
- Identification of Single-Use Applications
- Success Stories
- Semiconductor
- Electronic Marking Verification
- Counting Integrated Circuits
- Inspection of Two Barcodes
- Compact Safety Control
- Adhesive Detection on PCB Assembly
- Great Detail Inspection of Mobile Electronic Devices
- Error Proofing for IC Chips in Pocket Tape
- Presence and Orientation of IC Chips Seated in Nests
- Detection of Hard Disks
- Multiproduct Light-Guided Assembly Station
- Indication of FOUP Progress
- Safeguarding Small Access Points
- Success Stories


-turck-image.png)
-turck-image.png)

-turck-thumbnail.png)
-turck-thumbnail.png)