Your Global Automation Partner
Smart CAPA Management through RFID Sample Tracking for Pharma Quality Assurance
When it comes to the safety, quality and traceability of production processes, hardly any other industry has such demanding requirements as those in the pharmaceutical industry.
The extensive mandatory documentation and many other legal requirements must be observed at all times. The highest quality standards are therefore placed on data quality and sample tracking.
Regulatory bodies such as the FDA inspect pharmaceutical production sites unannounced. Enforcement actions in the event of regulatory violations range from the so-called FDA Warning Letter to the prohibition of a drug. With increasingly complex medical products, corrective and preventive actions (CAPA) help to ensure the highest quality standards in the pharmaceutical industry.
Contactless identification solutions based on RFID make it possible to maintain high safety standards and consistent documentation without the effort and error sources involved with manual systems – both in existing plants and new plants.
Turck's RFID systems ensure consistent sample tracking and optimize your production processes. They also ensure compliance with the ICH Q10 Guideline, which describes the requirements for a comprehensive pharmaceutical quality system and also includes Good Manufacturing Practice (GMP) requirements. You achieve end-to-end transparency with real-time data to ensure that your production is safe, efficient and standards compliant.
Turck's digital automation technologies maximum data integrity. Results outside the specification are thus excluded. Reproducible product quality, sustainable corrective and preventive actions (CAPA) and trouble-free GMP!
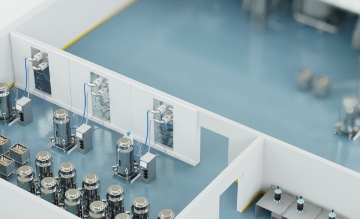
Safety: more CAPA thanks to digital solutions with RFID
From the tamper-proof monitoring of hose connections right through to reliable brand protection – Turck's powerful digital solutions considerably increase the efficiency of corrective and preventive actions. The use of state-of-the-art technologies ensures optimized production processes, prevents deviations and enables the targeted prevention of problems. In this way, we create the basis for sustainable quality assurance for the demanding pharma industry and boost your competitiveness.
Cost saving: HF bus mode in Ex Zone 1/21
Whether for couplings, paint cartridges, format or tool changes, RFID applications involving many read/write devices are often very expensive and time consuming to install and maintain due to the hardware required. With HF bus mode for its IP67 RFID interfaces, Turck has developed an efficient solution for this challenge. The function enables up to 32 HF read/write devices to be connected to each RFID input of an interface module. With four RFID channels per module, this means that up to 128 read points can be captured and parameterized centrally.
Efficiency: Complete track and trace solutions from a single source
With Turck, you benefit from turnkey track and trace solutions that not only include an intelligent RFID system but can also be flexibly extended with active tracking elements such as BLE (Bluetooth Low Energy), GPS or other IoT devices. Turck's GS1-compliantRFID solutions close the gap between physical production and the digital IT world of your company. We guarantee unique identities and reliable tracking for products, batches and more from production right through to shipping.
Effective Quality Assurance in the Pharmaceutical Industry with CAPA
Discover how our intelligent RFID systems take quality control to a new level in a wide range of pharmaceutical applications. From occupational safety to ensuring plant availability, increased productivity, reproducible quality and reliable brand protection – all from a single source.
Webinar: Quality Assurance (CAPA) in the Pharma Industry with RFID
RFID System Solutions for Pharma in Practice
In the application examples, see how users benefit from RFID system solutions in the pharmaceutical industry – in practical and vivid examples.
RFID Products and Solutions for the Pharma Industry:

RFID read/write devices (HF)
Turck's versatile HF RFID devices for demanding industrial requirements, including the Ex area or food applications (washdown, IP69K) enable optimum integration in your application. The read/write devices achieve ranges of up to one meter. Internationally standardized radio interface (ISO 15693, NFC type 5).

RFID reader (UHF)
Our UHF RFID read/write devices in a rugged industrial design (IP67) achieve ranges of several meters depending on the environment and can be perfectly integrated into your application. The globally standardized radio interface ISO 18000-6c and approvals for all relevant markets worldwide guarantee consistent solutions even for international customers.

HF RFID read/write devices for Ex Zone 1/21
Turck's TN-R42/TC-Ex is the world's only HF RFID read/write device available that is certified for direct use in ATEX Zone 1/21. The interface connection of the TN-R42/TC-Ex operates like a standard read/write device for the safe area. When used in conjunction with Turck's RFID interfaces, completely cabinet-free ID solutions for Ex areas can be implemented.

HF/UHF RFID interfaces
Turck's RFID interfaces link the read/write devices to the different networks such as OPC UA, PROFINET, EtherNet/IP, Modbus TCP, TCP/IP, PROFIBUS-DP, DeviceNet, CANopen and EtherCAT. The fully potted TBEN multiprotocol modules with RFID and I/Os, as well as the BL67 modular system in IP67 protection, are ideally suited for direct use in harsh environments – installed cabinet-free.
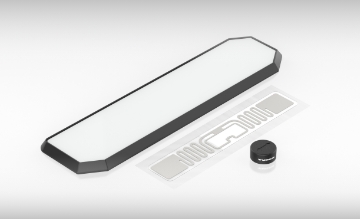
HF/UHF tags
Turck's tags comply with protection classes up to IP69K and are insensitive to contamination and liquids. They offer a reliable solution with memory sizes up to 8 Kbytes and a passive power supply. Select from a range of tags for high temperatures, direct mounting on metal, for the Ex area, autoclave applications and many others.
Turck Vilant Systems
Turck also offers complete turnkey RFID solutions through its subsidiary Turck Vilant Systems. The turnkey solutions specialist has already implemented more than 1,000 RFID system installations worldwide and increases the efficiency of all track and trace processes along the supply chain with reader systems, middleware developed in-house and onsite service:
Ask an Expert
If you have any questions about quality assurance in the pharmaceutical industry, our RFID solutions or a specific device, simply use our contact form. Our experts will be pleased to offer advice.


André Ammann










